GC – Gastric carcinoma
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
Introduction
In October 2021, the EMA granted marketing authorization for nivolumab in combination with fluoropyrimidine- and platinum-based chemotherapy for HER2-negative advanced or metastatic gastric adenocarcinoma in patients whose tumors express PD-L1 CPS ≥5.
Pembrolizumab is indicated in combination with trastuzumab and fluoropyrimidine- and platinum-based chemotherapy for the first-line treatment of locally advanced unresectable or metastatic HER2-positive adenocarcinoma of the gastroesophageal junction in adults with PD-L1-expressing tumours (CPS ≥ 1) and in combination with fluoropyrimidine- and platinum-based chemotherapy for the first-line treatment of locally advanced unresectable or metastatic HER2-negative adenocarcinoma of gastric or gastroesophageal junction in adults with PD-L1-expressing tumours (CPS ≥ 1).
Accordingly, we will determine CPS ≥ 1 and ≥ 5 for this entity.
Since December 2024, the EMA has approved tislelizumab, which can be used in combination with platinum- and fluoropyrimidine-based chemotherapy for the first-line treatment of locally advanced, unresectable or metastatic HER2-negative adenocarcinoma of the stomach in adults with a tumor area positivity (TAP) score of ≥ 5%.
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Gastrointestinal tract | |||||||||
| GC | 1L | ||||||||
| 2L | |||||||||
Last Update: 20. October 2025
Scores
CPS
Here, we are once again calculating the CPS – which we have already met in the context of HNSCCs, urothelial carcinomas, and carcinomas of the esophagus. As has already been stated on other pages in this Portal, the CPS is a cell score and stands for Combined Positive Score. For gastric carcinoma, the cut-off for the abovementioned therapy options is CPS ≥5.
For this score, we need to interpret not only staining in the membrane for carcinoma cells but also the positively stained lymphocytes and macrophages, which are then placed in relation to all viable carcinoma cells and multiplied by 100. The result is a calculated value – not a percentage.
While this value can be higher than 100 in principle, this is not found in practice in gastric carcinoma.
Granulocytes and plasma cells do not count towards the score.
Summed up, this results in the following calculation:
TAP
The TAP score is the ratio of the vital tumor and immune cells to the tumor area. We will also determine this score for adenocarcinoma from the gastro-oesophageal junction and squamous cell carcinoma of the oesophagus. The cut-off is the same for all entities at ≥ 5 %.
Please note that PD-L1 positive intra-luminal macrophages are not included in the evaluation unless they fill the lumen and are in direct contact with the carcinoma cells.
Interpretation guidance for gastric carcinoma
Use a high magnification: Since staining in the membrane may be very weak, the tissue under investigation should also always be screened at higher magnifications.
For differentiated signet ring cell adenocarcinomas in particular, the large intracytoplasmic mucin vacuoles can also cause difficulties in evaluation, leading to situations where the cytoplasm border displaced to the cell’s periphery is misinterpreted as staining in the membrane.
PD-L1 prevalence in gastric carcinoma
In current study data that include HER2-negative adenocarcinomas of the stomach, the gastroesophageal junction, and the esophagus, PD-L1 prevalence for nivolumab is found to be 60% relative to CPS ≥51.
For pembrolizumab, the prevalence in relation to CPS ≥ 1 is 88%, according to current studies that include HER2-positive adenocarcinomas of the stomach and gastro-oesophageal junction3 . For HER2-negative adenocarcinomas of the stomach and gastro-oesophageal junction, the prevalence is approx. 78%4.
The prevalence data for tislelizumab for locally advanced, unresectable or metastatic HER-2-negative adenocarcinoma of the stomach is 55%.
Test system validation/antibody selection in gastric carcinoma
The publication of a harmonization study performed in Germany is not yet final. In terms of the study antibodies, it can be seen that, for the squamous cell carcinomas and AEG tumors investigated, as has already been shown with other entities, SP142 stains comparatively fewer tumor cells than the other antibodies. On the other hand, SP263 provides us with a stronger expression pattern overall.
In a published harmonization study is described that the 28-8, 22C3 and SP263 antibody assays achieve comparable staining results for the determination of the TAP score6.
Guidance for practice
Specific interpretation guidance for gastric carcinoma
Support for interpretation is available from Agilent DAKO2 at the following link: Agilent DAKO Interpretation Handbook
Please note the following:
- A least 100 carcinoma cells should be available for interpretation.
- Necrotic cells, plasma cells, and granulocytes are excluded from CPS and TPS scoring.
- When evaluating esophageal cancer, a carcinoma in situ component is excluded from CPS scoring.
The following tables provide you with full details and guidance for interpreting the CPS score. (Agilent DAKO 4, page 26)
Interpretation guidance for CPS definition numerator
| Tumor cells | Immune cells | Other cells | |
| Included in numerator | Convincing partial or complete linear membrane staining (at any intensity) of viable invasive tumor cells | Membrane and/or cytoplasmic* staining (at any intensity) of mononuclear inflammatory cells (MICs) within tumor nests and adjacent supporting stroma
| Excluded |
| Excluded from numerator |
|
|
|
* In MICs, membrane and cytoplasmic stainings often cannot be distinguished due to the high nucleus-plasma relation. For this reason, both membrane and cytoplasmic stained MICs are contained in the score.
† “Neighboring MICs” is the definition of cells that are inside the same 20x field as the tumor. However, MICs that are not directly associated with the reaction to the tumor should be ruled out.
‡ Macrophages and histiocytes are assigned to the same kind of cell.
The following tables provide you with full details and guidance for interpreting the CPS score. (Agilent DAKO 5, page 24)
Interpretation guidance for CPS definition denominator
| Tumor cells | Immune cells | Other cells | |
| Included in denuminator | All viable invasive tumor cells | Excluded | Excluded |
| Excluded from denuminator |
| All immune cells of any type |
|
Interpretation guidance for TAP definition
The Tumor Area Positivity Score (TAP) is described as the total percentage of tumor area (tumor and desmoplastic tumor-associated stroma) covered by tumor cells and tumor-associated immune cells with PD-L1 staining of any intensity. In contrast to TPS and TC are these cells in relation to an area. This score, like the IC, is an area score5.
QuIP Proficiency Test PD-L1 GC
Criteria for the proficiency test PD-L1 Gastric carcinoma
The proficiency test PD-L1 Gastric carcinoma requires only one Score, the CPS-Score.
Cut-Offs of the proficiency test PD-L1 Gastric carcinoma
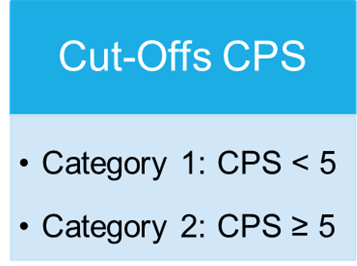
Overview
In the proficiency test, only the Combined Positive Score (CPS) is required, as in clinical routine the determination of the CPS is decisive for the first-line use of Pembrolizumab. In the proficiency test, the determination of the CPS-Score is conducted via immunohistochemical staining. The Score is defined as the quotient of positively stained tumour cells, lymphocytes and macrophages in relation to all vital tumour cells multiplied by 100. The result is not a percentage value.
The participants should evaluate the cases presented with regard to the applicable cut-offs and classify them accordingly.
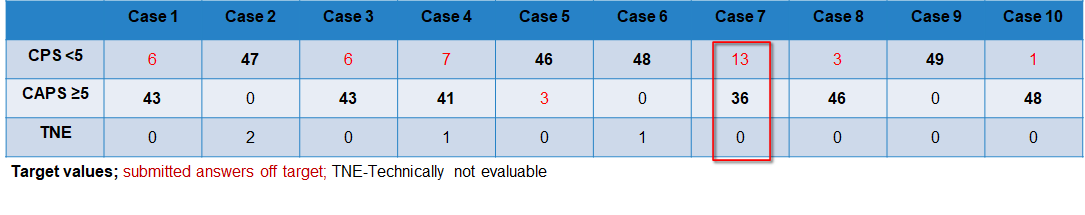
Case-related result submissions of the participants
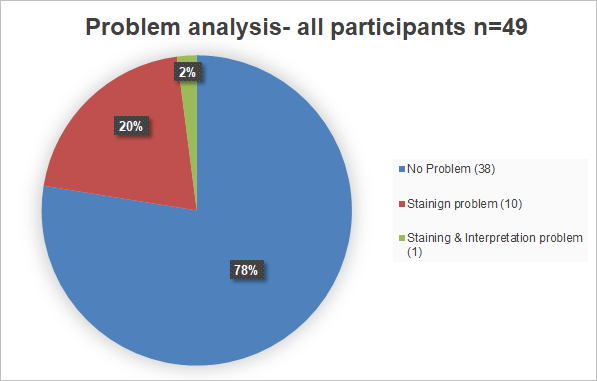
The breakdown of participant inputs per case is in the following table. The case 7 caused problems for at lot of participants. 26,5 % of the participants entered incorrect submissions for case 7. After a thorough review of the slides points were still given if staining and interpretation were appropriate. Those participants that did not receive any points after the review mostly had staining problems.
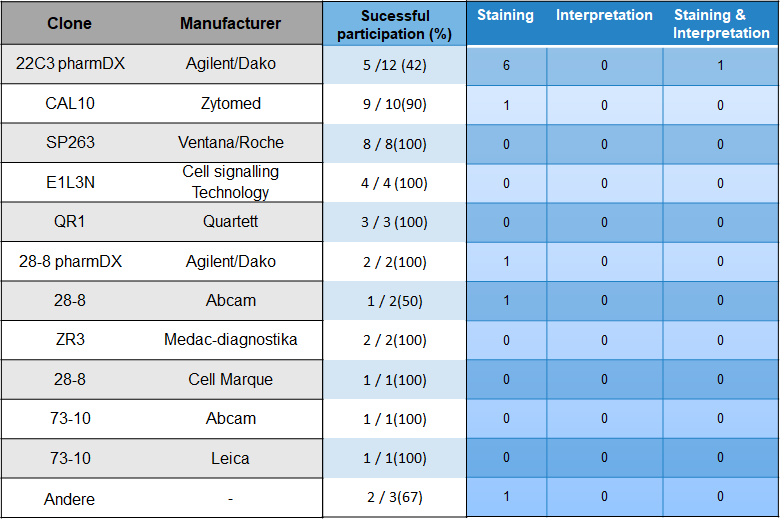
Choice of antibody and problem analysis
With 24 % most participants used the antibody clones 22C3 from Agilent/Dako. Followed by CAL10 from Zytomed (20%) and SP263 from the manufacturer Ventana/Roche (163,3.
Pass rates
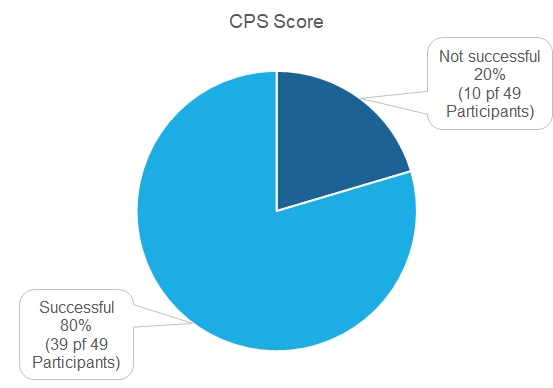
49 Institutions did partake in the proficiency test of which 39 (80 %) were successful. 10 Institutions were not successful (20 %).
Problem analysis
Overall does the problem analysis confirm the good passing rate. Participants with problems had mainly staining problems.
Literature
1. Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet 398, 27–40 (2021).
2. Agilent Technologies Inc. PD-L1 IHC 28-8 pharmDx Interpretation Manual–Gastric Adenocarcinoma, Gastroesophageal Junction (GEJ) Adenocarcinoma, and Esophageal Adenocarcinoma. PD-L1 IHC 28-8 pharmDx is CE-IVD marked for in vitro diagnostic use. (2021).
3. Janjigian et al., Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. The Lancet 402,2197-2208 (2023)
4.Rha et al., Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology 24,11, 1181-1195 (2023)
5. Qiu et al: Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial BMJ;385:e078876 | doi: 10.1136/bmj-2023-078876 (2024)
6. Klempner et al: PD-L1 Immunohistochemistry in Gastric Cancer: Comparison of Combined Positive Score and Tumor Area Positivity Across 28-8, 22C3, and SP263 Assays. JCO precision oncology, Volume 8, https://doi.org/10.1200/PO.24.00230 (2024)


